By applying the principles of Quality-by-Design (QBD), EDELWEISS PHARMA partners with formulation experts to develop several conventional and specialised dosage forms. We provide support at all stages of drug development to find a suitable dosage form to suit your marketing needs.
Our Drug Product Development services are listed below.
Pre-formulation studies:
Robust pre-formulation studies are essential for successful development of dosage form(s) to suit the intended purpose at pre-clinical or clinical stage(s).
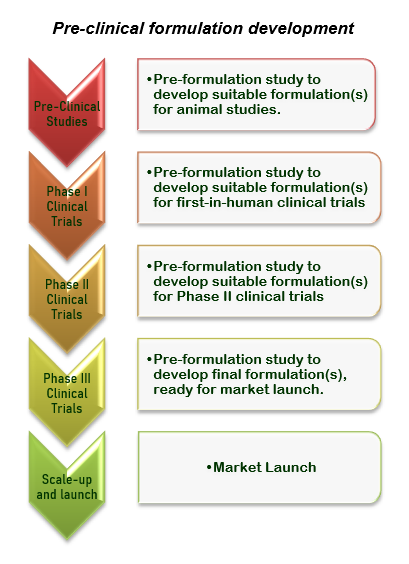
Pre-formulations studies includes the following.
- Excipient compatibility
- Stress studies
- Stability studies
- Accelerated stability studies
- Polymorphism
- Dissolution
- Hygroscopicity
- Physicochemical properties (eg. Solubility, bulk and tapped density)
Formulation Development
Expertise in formulation development includes:
- IR tablets
- MR / CR tablets
- IR capsules
- MR / CR capsules
- Oral solutions, suspensions and powders for reconstitution
- Effervescent tablets and powders
- Topical preparations (eg. Ointments, creams and lotions)